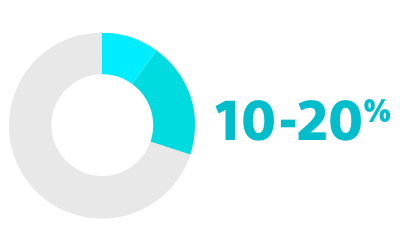
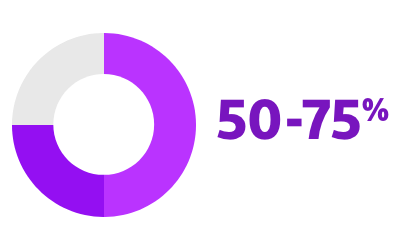
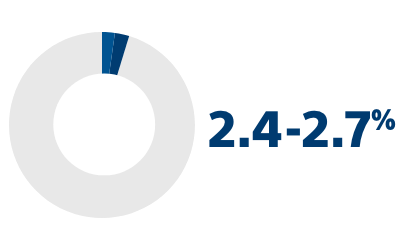
RISK OF PROGRESSION FROM PAROXYSMAL AF TO PERSISTENT OR PERMANENT FORMS2
- The CABANA randomised controlled trial (n=2,204) demonstrated that catheter ablation is more effective than antiarrhythmic drugs (AADs) in reducing recurrence of AF1
*AADs: Antiarrhythmic drugs
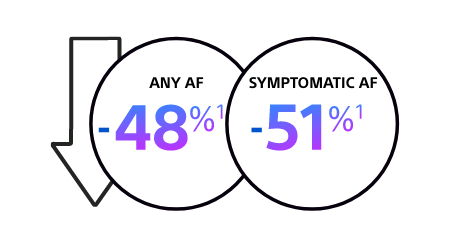
CABANA TRIAL: RECURRENCE OF AF
Catheter ablation is associated with a significant reduction in recurrence of symptomatic AF and any AF1
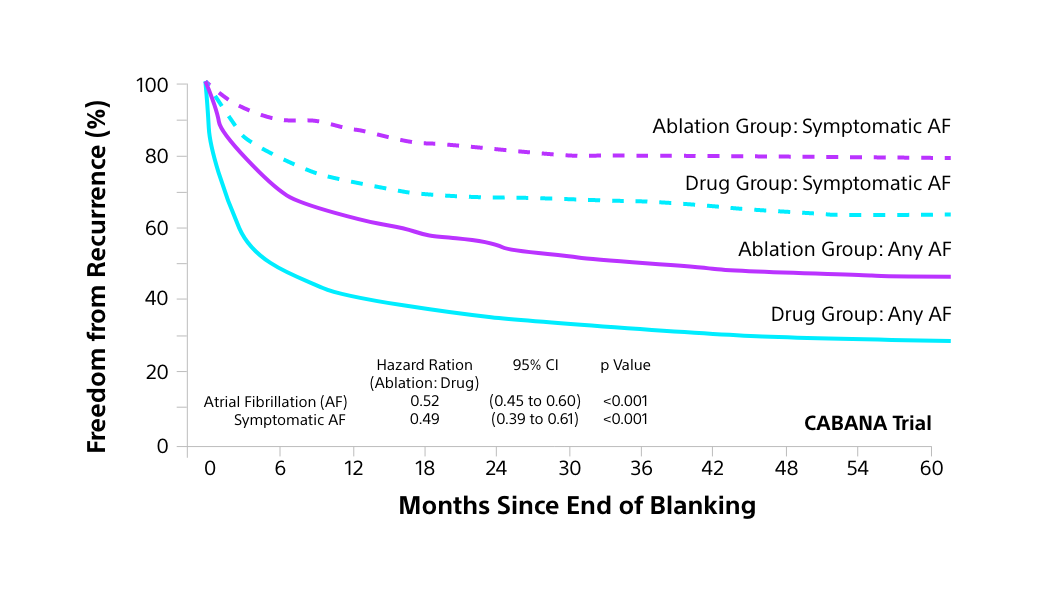
Blanking Period: The blanking period is the first 3 months after AF ablation when arrhythmias may occur but are not counted as treatment failure, reflecting healing and remodelling.
- Meta-analyses of published studies also show the following benefits of catheter ablation vs. drug therapy:1,5-12
ESC Recommendations: catheter ablation
Given the strength of evidence supporting catheter ablation for the treatment of AF, it is recommended by European Society of Cardiology (ESC) as a first-line treatment strategy for paroxysmal AF; catheter ablation is also advised for certain other AF patients.13
FIRST-LINE RHYTHM CONTROL THERAPY
Classa
Levelb
Catheter ablation is recommended as a first-line option with a shared decision-making rhythm control strategy in patients with paroxysmal AF (Atrial Fibrillation), to reduce symtoms, recurrence, and progression of AF.
1
A
AF PATIENTS RESISTANT OR INTOLERANT TO ANTIARRHYTHMIC DRUG THERAPY
Classa
Levelb
Catheter ablation is recommended in patients with paroxysmal or persistent AF resistant or intolerant to antiarrhythmic drug therapy to reduce symptoms, recurrence, and progression of AF.
1
A
PATIENTS WITH HEART FAILURE
Classa
Levelb
AF catheter ablation is recommended in patients with AF and HFrEF (Heart Failure with reduced Ejection Fraction) with high probability of tachycardia-induced cardiomyopathy to reverse left ventricular dysfunction.
1
B
Table adapted from Recommendation Table 18, 2024 ESC Guidelines for the Management of Atrial Fibrillation.13
aClass of recommendation
bLevel of evidence
NICE guidelines
NICE guidelines for the treatment of AF (2021)14 do not currently align with this more recent guidance from the ESC (2024)13 or guidance in the United States (2023),15 which both prioritise early rhythm control and support catheter ablation for the treatment of AF as a first-line therapy. However, NICE guidance does recommend:
OFFERING PATIENTS A PERSONALISED PACKAGE OF CARE14
REFERRING PROMPTLY AT ANY STAGE IN TREATMENT IF IT FAILS TO CONTROL THE SYMPTOMS AND MORE SPECIALISED MANAGEMENT IS NEEDED
(within 4 weeks of failed treatment or recurrence of AF after cardioversion)14
Why FARAPULSE™ pulsed field ablation (PFA) vs “thermal” ablation?
Confidence in rigorous clinical research
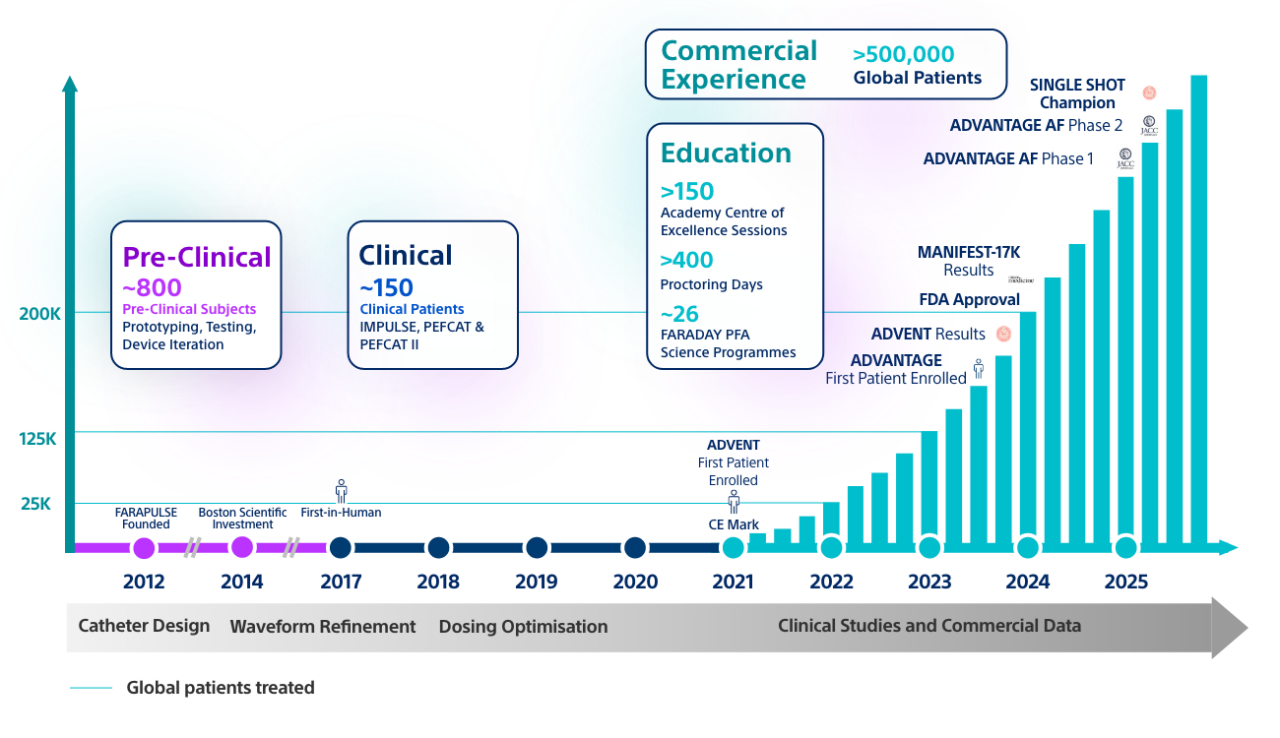
FARAPULSE is backed by over 10 years of research and over 150 publications including 55 clinical trials.16,17
FARAPULSE PFA: Backed by >10 years of research
AF: Atrial fibrillation; PFA: Pulsed field ablation
A NICE-recommended treatment
Following an evidence-based review, NICE guidance now recommends PFA as an option for the treatment of AF39.
FARAPULSE PFA: demonstrated safety
74,390 patients Treated in Published Clinical Trials/Registries
8 real-world registries globally
Low rates of adverse events
0.63%
0.12%
MAJOR ADVERSE EVENT RATE
STROKE RATE
No reported thermal complications
- In MANIFEST-17K, there were zero thermal complications across all >17,000 procedures with FARAPULSE PFA24
FARAPULSE PFA: demonstrated efficacy
- In the ADVENT US IDE RCT, FARAPULSE PFA demonstrated:
- A high primary effectiveness rate of 73.3%
- Non-inferiority to thermal ablation for primary efficacy outcomes22
- Significantly reduced atrial arrhythmia (AA) burden* vs. thermal technologies. Residual AA burden is gaining acceptance as a more comprehensive measure of ablation efficacy, with a burden of >0.1% linked to impaired quality of life and higher health care utilisation (HCU)23,27,28
- A sub-analysis of the ADVENT RCT found that, at 12-months, more patients had an AA burden of <0.1% with FARAPULSE PFA than with thermal technologies23
*AA burden is defined as the percentage of time the patient was in atrial arrhythmia during a defined time.
FARAPULSE PFA: IMPROVED REDUCTION IN AA BURDEN VS. THERMAL ABLATION23
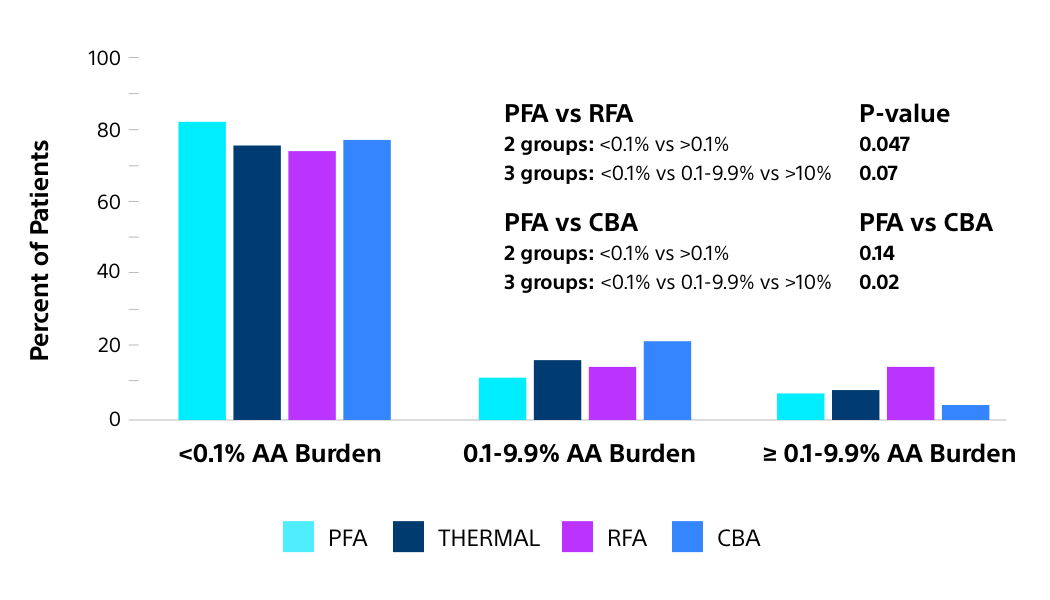
THIS DIFFERENCE IS CLINICALLY MEANINGFUL IN TERMS OF BOTH PATIENT QUALITY OF LIFE AND HEALTHCARE UTILISATION

QUALITY OF LIFE (QoL)
Significantly greater QoL improvement in patients with AA burden <0.1% vs. ≥10%.23
HEALTHCARE UTILISATION
Significantly lower risk for redo ablation, cardioversion and hospitalisation with aa burden <0.1% vs. ≥0.1%.23
ABLATION MODALITY
FARAPULSE PFA patients significantly more likely to have AA burden <0.1% than radiofrequency ablation23
High real-world rates of freedom from recurrence
- FARAPULSE has consistently demonstrated high one-year freedom from recurrence rates, consistent across numerous real-world study designs of various sizes, confirming the results from the ADVENT randomised controlled trial.
CONSISTENTLY HIGH RATES OF ONE-YEAR FREEDOM FROM RECURRENCE
FOR FARAPULSE PFA IN REAL-WORLD STUDIES
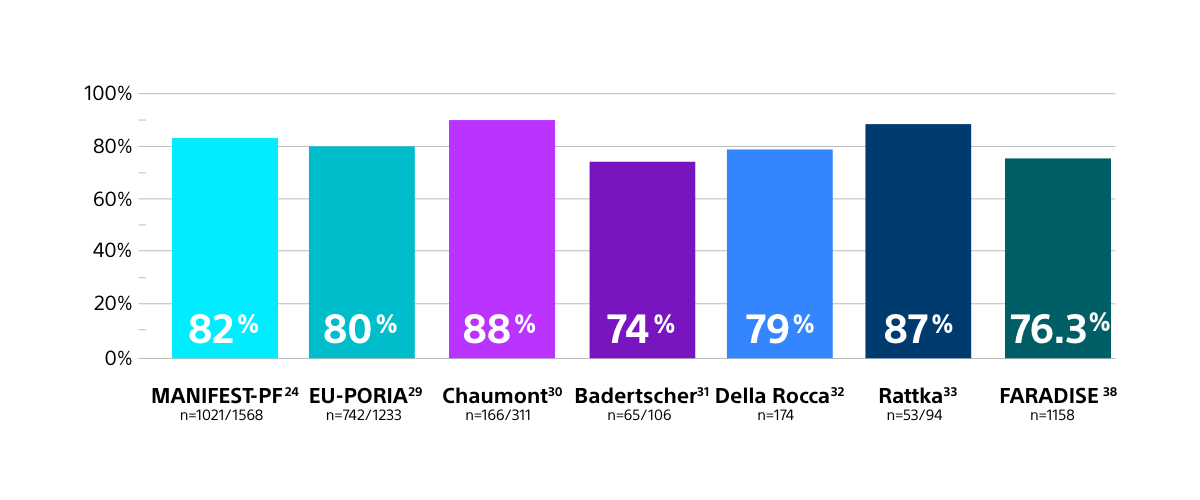
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
- Across multiple independent comparative studies, reflecting real-world practice, FARAPULSE patients have trended towards a lower rate of arrhythmia recurrence at one year compared to thermal ablation, confirming the results from the ADVENT randomised controlled trial.
FAVOURABLE RATES OF FREEDOM FROM AF FOR FARAPULSE PFA
COMPARED TO THERMAL ABLATION IN INDEPENDENT STUDIES
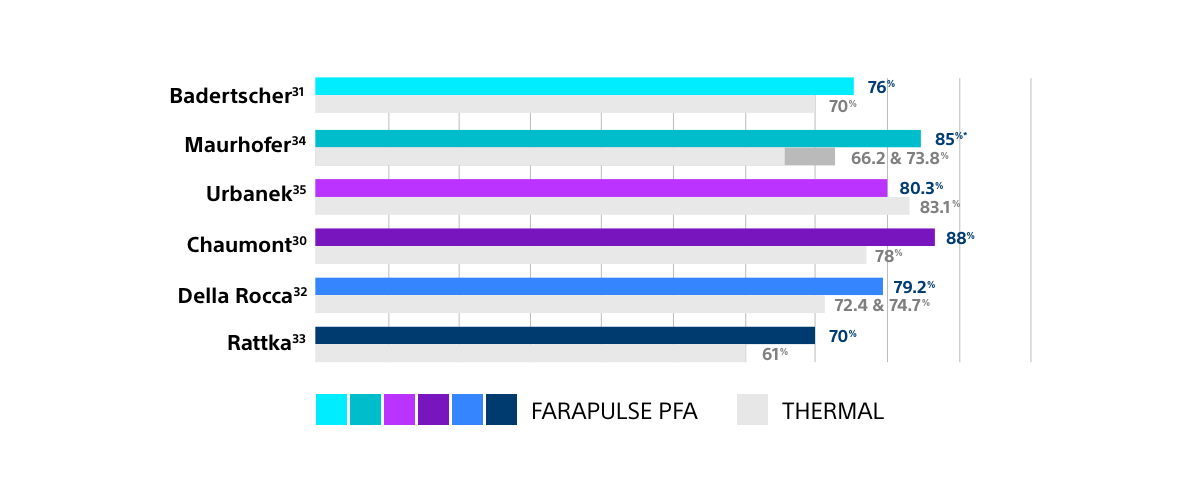
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
FARAPULSE PFA PROCEDURES TYPICALLY LAST AROUND 1 HOUR OR LESS
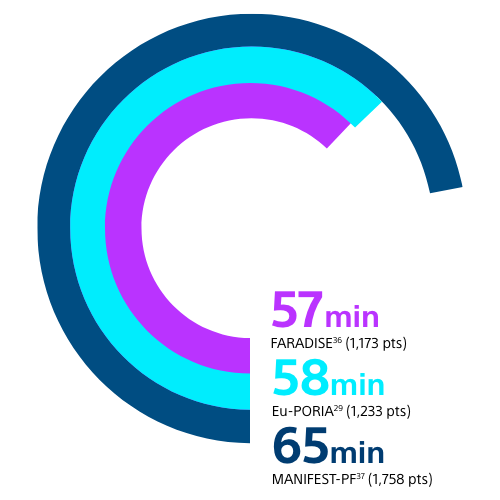
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
In addition to these procedural efficiencies, a reduced AA burden after treatment, as seen with FARAPULSE PFA vs thermal ablation, has been shown to be associated with reduced healthcare utilisation (HCU), including all-cause hospitalisations and need for redo procedures.23,28
HCU THROUGH 12 MONTHS BY AA BURDEN
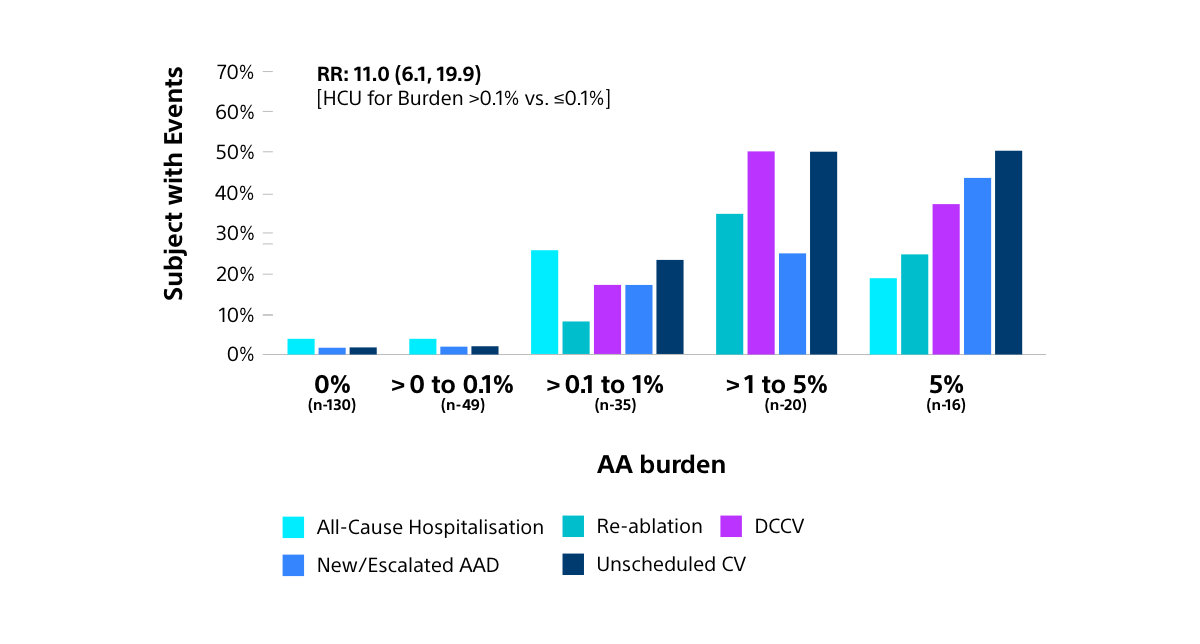
AA: Atrial arrhythmia; AAD: Antiarrhythmic drug; CV: Cardioversion; DCCV: Direct current cardioversion; HCU: Healthcare utilisation; RR: Relative ratio
IMPULSE, PEFCAT, PEFCAT II20,21
First-In-Human Trials
Collectively demonstrated the system's safety and effectiveness in treating Atrial Fibrillation.
ADVENT
Established the safety and efficacy of the FARAPULSE PFA system in patients with paroxysmal AF.
ADVANTAGE AF PHASE 1 & 218
Evaluating FARAPOINT™ focal linear PFA Catheter’s* safety and efficacy to ablate CTI as an adjunctive device when used with FARAWAVE™ to ablate the pulmonary veins and posterior wall.
*Currently not approved for commercial use
CTI: Cavotricuspid isthmus
DISCOVER MORE ABOUT FARAPULSE
READY TO DISCUSS FARAPULSE WITH YOUR PATIENTS?
Pipeline of key clinical trials
BOSTON SCIENTIFIC TRIALS
FARADISE
1000+ pt prospective registry (NCT05501873)
FARAWAY
FARAPULSE Workflow Assessment Registry (NCT06656884)
OPALISE
A Registry on the FARAVIEWTM Technology of the OPAL HDxTM Mapping System When Used With the FARAWAVE NAV Ablation Catheter in the Treatment of Atrial Fibrillation (NCT06808217)
AVANT GUARD
1st line PFA vs. AAD in persistent AF w/ILR (NCT06096337)
DISRUPT-AF
Registry and randomised workflow study (NCT06335082)
NAVIGATE PF FARAVIEW
Nav-enabled mapping integration (NCT06175234)
ADVENT PAS
FDA-mandated PMCF (NCT06431815)
ADVENT LTO
(3-yr) Long-term follow-up of PFA vs. thermal ablation (NCT06526546)
OPTION-A
Concomitant WATCHMAN™ + FARAPULSE (NCT06686485)
REMATCH
Redo AF with FARAPOINT focal-linear PFA catheter (NCT06735534)
INDEPENDENT STUDIES
CABA HFpEF
Ablation for heart failure patients vs. medical therapy (NCT05508256)
BEAT AF
Compare RFA vs. PFA for paroxysmal and persistent AF patients (NCT05159492)
FACIL AF
Compare PFA vs. cryoablation for paroxysmal AF (NCT05940597)
REPEAT AF
PFA for redos AF (NCT06199180)
DOPPIO
Ablation strategy with PFA for PVI (NCT07021313)
VEMAPULSE
Ablation strategy for persistent AF and comparison with Marshall plan (NCT06383975)
France PFA Cohort
French registry on PFA for AF (NCT06497933)
AAD: Antiarrhythmic drugs; AF: Atrial fibrillation; EMEA: Europe, Middle East & Africa; FDA: Food and Drug Administration; ILR: Implantable loop recorder; PFA: Pulsed field ablation; PMCF: Post-market clinical follow-up; PVI: Pulmonary vein isolation RFA: Radiofrequency ablation
REFERENCES:
1. Poole JE, Bahnson TD, Monahan KH, et al. CABANA Investigators and ECG Rhythm Core Lab. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol. 2020 Jun 30;75(25):3105–3118. doi: 0.1016/j.jacc.2020.04.065.
2. Proietti R, Hadjis A, AlTurki A, et al. “A systematic review on the progression of paroxysmal to persistent atrial fibrillation: shedding new light on the effects of catheter ablation.” JACC Clin Electrophysiol. 2015 Jun;1(3):105-115. doi: 10.1016/j.jacep.2015.04.010
3. Benali K, Macle L, Haïssaguerre M, et al. Impact of Catheter Ablation of Atrial Fibrillation on Disease Progression. J Am Coll Cardiol EP. 2025 ;11(2) :421–435.doi:10.1016/j.jacep.2024.10.017.
4. Karakasis P, Tzeis S, et al. Impact of catheter ablation timing according to duration of atrial fibrillation history on arrhythmia recurrences and clinical outcomes: A meta-analysis. Europace. 2025 May 28:euaf110. doi: 10.1093/europace/euaf110.
5. AlTurki A, Proietti R, Dawas A, et al. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a symptomatic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19(1):18. doi: 10.1186/s12872-019-0998-2.
6. Asad ZUA, Yousif Ali, Khan MS, et al. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ Arrhythm Electrophysiol. 2019;12(9):e007414. doi: 10.1161/CIRCEP.119.007414.
7. Khan SU, Rahman H, Talluri S, et al. The Clinical Benefits and Mortality Reduction Associated With Catheter Ablation in Subjects With Atrial Fibrillation: A Systematic Review and Meta-Analysis JACC Clin Electrophysiol. 2018;4(5):626-635. doi: 10.1016/j.jacep.2018.03.003
8. Ruzieh M Foy AJ, Aboujamous NM, et al. Meta-Analysis of Atrial Fibrillation Ablation in Patients with Systolic Heart Failure. Cardiovasc Ther. 2019:8181657. doi: 10.1155/2019/8181657.
9. Turagam MK, Garg J, Whang W, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170(1):41-50. doi: 10.7326/M18-0992
10. Chen C, Zhou X, Zhu M, et al. Catheter ablation versus medical therapy for patients with persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized controlled trials. J Interv Cardiovasc Electrophysiol. 2018;52(1):9-18. doi: 10.1007/s10840-018-0349-8
11. Calkins H, Hindricks G, Cappato R, et al. Heart Rhythm. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi: 10.1016/j.hrthm.2017.05.012.
12. Allan KS, Aves T, Henry S, et al. Health-Related Quality of Life in Patients with Atrial Fibrillation Treated with Catheter Ablation or Antiarrhythmic Drug Therapy: A Systematic Review and Meta-analysis. CJC Open. 2020;2(4):286-295. doi: 10.1016/j.cjco.2020.03.013.
13. Van Gelder IC, Rienstra M, Bunting KV, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314-3414. doi:10.1093/eurheartj/ehae176
14. NICE Guideline NG196. Atrial fibrillation: diagnosis and management. April 2021, updated June 2021 available at https://www.nice.org.uk/guidance/ng196/chapter/Recommendations (accessed April 2025)
15. Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193
16. BSC data on file
17. FARAPULSE Clinical Compendium.
18. A Prospective Single Arm Open Label Study of the FARAPULSE Pulsed Field Ablation System in Subjects with Persistent Atrial Fibrillation (ADVANTAGE AF). ClinicalTrials.gov ID: NCT05443594
19. Pulsed Field Ablation (PFA) vs Anti-Arrhythmic Drug (AAD) Therapy as a First Line Treatment for Persistent Atrial Fibrillation (AVANT GUARD). ClinicalTrials.gov ID: NCT06096337
20. Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7(5): 614-627. doi: 10.1016/j.jacep.2021.02.014
21. Musikantow D, Neuzil P, Anic A, et al. Long-Term Clinical Outcomes of Pulsed Field Ablation in the Treatment of Paroxysmal Atrial Fibrillation. J Am Coll Cardiol EP; 9(9):2001–2003. doi: 10.1016/j.jacep.2023.06.019.
22. Reddy VY, Gerstenfeld EP, Natale A, et al; ADVENT Investigators. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2023;389(18):1660-1671. doi: 10.1056/NEJMoa2307291.
23. Reddy V, Mansour M, Calkins H, et al. Pulsed Field vs Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation: Recurrent Atrial Arrhythmia Burden. J Am Coll Cardiol. 2024 Jul 2;61-74. doi: 10.1016/j.jacc.2024.05.001
24. Ekanem E, Neuzil, P, Reichlin T, et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med (2024). doi:10.1038/s41591-024-03114-3.
25. Reddy V, et al. 2025.A Prospective Single Arm Open Label Study of the FARAPULSE Pulsed Field Ablation System in the subjects with Persistent Atrial Fibrillation (ADVANTAGE AF). AF Symposium 2025, Boston, MA, USA.
26. Reddy VY, Gerstenfield EP, Schmidt B, et al. Pulsed Field Ablation of Persistent Atrial Fibrillation With Continuous ECG Monitoring Follow-Up: ADVANTAGE AF-Phase 2. Circulation. Published online April 2025. Doi:10.1161/CIRCULATIONAHA.125.074485
27. Reichlin T, Kueffer T, Badertscher P, et al. Pulsed Field or Cryoballoon Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2025;392:1497-1507. doi: 10.1056/NEJMoa2502280
28. Andrade JG, Deyell MW, Macle L, et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCADOSE study. Eur Heart J 2023; 44: 765-76.
29. Schmidt B, Bordignon S, Nevenet K, et al. European real-world outcomes with pulsed field ablation in patients with symptomatic atrial fibrillation: Lessons from the multi-centre EU-PORIA Registry. Europace. 2023;25(7):euad185.
30. Chaumont C, McDonnell E, Boveda S, et al. Prospective 1-year results of atrial fibrillation ablation using the pentaspline pulsed field ablation catheter: The initial French experience. Arch Cardiovasc Dis. 2024;117(4):249-254. doi:10.1016/j.acvd.2024.01.005.
31. Badertscher P, Weidlich S, Knecht S, et al. Efficacy and safety of pulmonary vein isolation with pulsed field ablation vs. novel cryoballoon ablation system for atrial fibrillation. Europace 2023;25(12):euad329. doi: 10.1093/europace/euad329.
32. Della Rocca DG, Marcon L, Magnocavallo M, et al. Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: A propensity scorematched comparison. Europace. 2023;26(1):euae016. doi: 10.1093/europace/euae016.
33. Rattka M, Mavrakis E, Vlachopoulou D, et al. Pulsed field ablation and cryoballoon ablation for pulmonary vein isolation: Insights on efficacy, safety and cardiac function. J Interv Card Electrophysiol. 2024;67(5):1191-1198.
34. Maurhofer J, Kueffer T, Madaffari A, et al. Pulsed-field vs. cryoballoon vs. radiofrequency ablation: A propensity score matched comparison of one-year outcomes after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2024;67(2):389-397. doi: 10.1007/s10840-023-01651-4
35. Urbanek L, Bordignon S, Schaack D, et al. Pulsed field versus cryoballoon pulmonary vein isolation for atrial fibrillation: Efficacy, safety, and long-term follow-up in a 400-patient cohort. Circ Arrhythm Electrophysiol. 2023;16(7):389-398. doi: 10.1161/CIRCEP.123.011920
36. Boresma LV, Szeplaki G, Garcia-Bolao I, et al. Real World Data Collection in Subjects Treated with the FARAPULSE Pulsed Field Ablation System (FARADISE). Heart Rhythm 2024;21(7):1197. doi:10.1016/j.hrthm.2024.04.031
37. Ekanem E, Reddy VY, Schmidt B, et al., Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF) [published correction appears in Europace. 2023 Feb 16;25(2):449. doi:10.1093/europace/euac250]. Europace. 2022;24(8):1256-1266. doi:10.1093/europace/euac050
38. Lucas V A Boersma et al. On Behalf of the FARADISE Investigators, Real-world experience with the pentaspline pulsed field ablation system: one-year outcomes of the FARADISE registry, EP Europace, Volume 27, Issue 9, September 2025, euaf182, https://doi.org/10.1093/europace/euaf182
39. NICE guidance IPG806: Pulsed-field ablation for atrial fibrillation. Last accessed October 2025.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
Certain components are pending CE Mark, not available for sale in the European Economic Area (EEA).
This material not intended for use in France.
Pending CⲈ MARK