A wealth of evidence, including 55 clinical trials and more than 150 scientific publications to date,1 has established the excellent safety and effectiveness of FARAPULSE PFA for the treatment of Atrial Fibrillation (AF), and the potential advantages of this approach versus thermal ablation. Outcomes from multiple clinical trials demonstrate that treatment with FARAPULSE PFA offers:
- Minimal risk of damage to adjacent structures2-5
- Low rates of procedural complications2-5
- High freedom from arrhythmia recurrence, and low rates of repeat ablation2,3,8
- Proven non-inferiority in terms of safety and efficacy compared to thermal ablation techniques,² with superior efficacy vs. cryoballoon ablation demonstrated in one randomised controlled trial9
- Minimal atrial arrhythmia (AA) burden* post ablation6,7,9
- A randomised controlled trial has shown a significant reduction compared to thermal ablation7
- An AA burden under a certain threshold appears to translate into improved quality of life7,9,10
*AA burden is defined as the percentage of time a patient in in atrial arrhythmia during a defined time period and is increasingly recognised as a more comprehensive measure of ablation efficacy, with a burden >0.1% linked to impaired quality of life and higher healthcare utilisation.7,9,10
- Significantly higher durability of pulmonary vein isolation8
- A shorter learning curve and confidence in more reproducible results for the operator3,11,12
- Shorter procedure times2,9,11
In addition, many patients experience minimal pain and report fast recovery times.13
FARAPULSE PFA in the treatment of AF
A NICE-recommended treatment
Following an evidence-based review, NICE guidance now recommends PFA as an option for the treatment of AF14.
SAFE
<1%
RATE OF MAJOR ADVERSE EVENTS3
0
REPORTS OF OESOPHAGEAL FISTULA OR DYSMOTILITY, PULMONARY VEIN STENOSIS, OR PERSISTENT PHRENIC NERVE INJURY3
EFFECTIVE
LOW RATES OF ARRHYTHMIA RECURRENCE1,2,10,15
MINIMAL ATRIAL ARRYTHMIA (AA) BURDEN*6
*A reduced AA burden appears to translate to improved QoL
PROVEN
500k PATIENTS TREATED16
55 CLINICAL TRIALS1
150+ PUBLICATIONS1
EFFICIENT
PROCEDURE TIME TYPICALLY ~1 HOUR OR LESS11,17,18
Positive impact on quality of life6,16
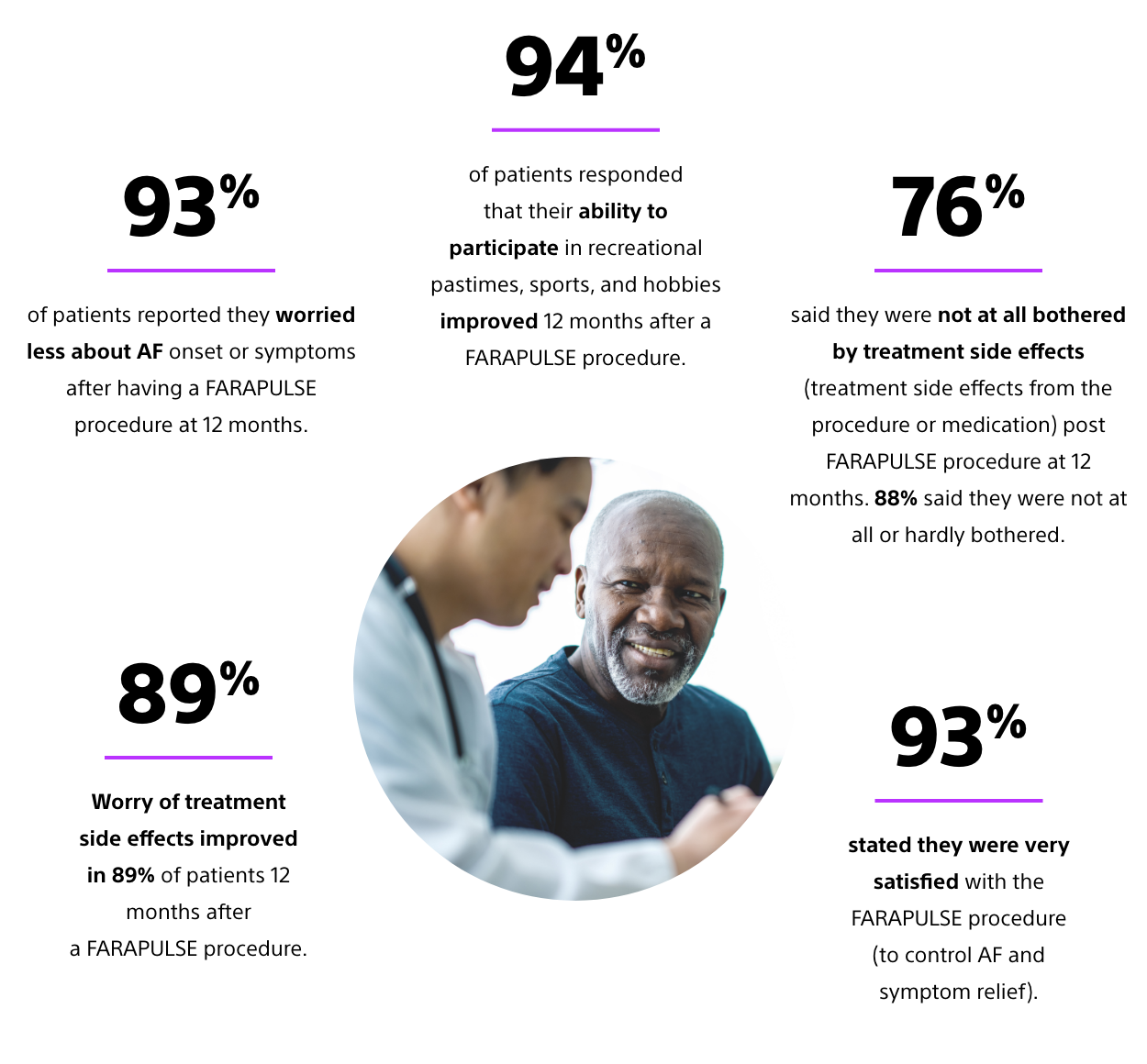
Data taken from ADVENT randomised clinical trial AFEQT (Atrial Fibrillation Effect on QualiTy of life). AFEQT is a series of questions asked to patient at baseline and at 12 months post procedure. Quality-of-life scores improved post-ablation compared to baseline.
REFERENCES:
1. FARAPULSE Clinical compendium.
2. Reddy VY, Gerstenfeld EP, Natale A, et al, ADVENT Investigators. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2023;389(18):1660-1671. doi: 10.1056/NEJMoa2307291.
3. Ekanem E, Neuzil P, Reichlin T, et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med. 2024;30(7):2020-2029. doi: 10.1038/s41591-024-03114-3
4. Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II JACC: Clinical Electrophysiology 2021;7(5): 614-627. doi: 10.1016/j.jacep.2021.02.014
5. Schmidt B, Bordignon S, Tohoku S, et al. 5S Study: Safe and Simple Single Shot Pulmonary Vein Isolation with Pulsed Field Ablation Using Sedation. Circ Arrhythm Electrophysiol. 2022;15(6): e010817. doi: 10.1161/CIRCEP.121.010817
6. Reddy VY, Gerstenfield EP, Schmidt B, et al. Pulsed Field Ablation of Persistent Atrial Fibrillation with Continuous ECG Monitoring Follow-Up: ADVANTAGE AF-Phase 2. Circulation. Published online April 2025. Doi:10.1161/CIRCULATIONAHA.125.074485
7. Reddy V, Mansour M, Calkins H, et al. Pulsed Field vs Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation: Recurrent Atrial Arrhythmia Burden. J Am Coll Cardiol. 2024;84(1):61-74. doi:10.1016/j.jacc.2024.05.001
8. Della Rocca DG, Marcon L, Magnocavallo M, et al. Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: A propensity score matched comparison. Europace. 2023;26(1):euae016. doi: 10.1093/europace/euae016
9. Reichlin T, Kueffer T, Badertscher P, et al. Pulsed Field or Cryoballoon Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med 2025;392:1497-1507. doi: 10.1056/NEJMoa2502280
10. Andrade JG, Deyell MW, Macle L, et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCADOSE study. Eur Heart J 2023; 44: 765-76.
11. Schmidt B, Bordignon S, Neven K, et al. European Real-World Outcomes with Pulsed Field Ablation in Patients with Symptomatic Atrial Fibrillation - Lessons from the Multicenter EU-PORIA Registry. Europace 2023;25(7):euad185. doi: 10.1093/europace/euad185
12. 1-year outcomes from the MANIFEST-PF registry, presented by Vivek Y. Reddy, MD at EHRA 2023
13. Füting A, Neven K, Howel D, et al. Patient discomfort following pulsed field ablation for paroxysmal atrial fibrillation – an assessment of chest and groin pain using the Numeric Rating Scale. Clin Res Cardiol (2021). doi:10.1007/s00392-021-01933-9
14. NICE guidance IPG806: Pulsed-field ablation for atrial fibrillation. Last accessed July 2025.
15. Ruwald MH, Johannessen A, Hansen ML, et al. Pulsed field ablation in real-world atrial fibrillation patients: clinical recurrence, operator learning curve and re-do procedural findings. J Interv Card Electrophysiol. 2023;66(8):1837-1848. doi: 10.1007/s10840-023-01495-y.
16. Boston Scientific data on file.
17. Ekanem E, Reddy VY, Schmidt B, et al., Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF) [published correction appears in Europace. 2023 Feb 16;25(2):449. doi:10.1093/europace/euac250]. Europace. 2022;24(8):1256-1266. doi:10.1093/europace/euac050
18. Boresma LV, Szeplaki G, Garcia-Bolao I, et al. Real World Data Collection in Subjects Treated with the FARAPULSE Pulsed Field Ablation System (FARADISE). Heart Rhythm 2024;21(7):1197. doi:10.1016/j.hrthm.2024.04.031
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.