Treatment options
Cardioversion

Electrical or pharmacological cardioversion may be used to restore sinus rhythm, particularly in patients with acute or worsening haemodynamic instability. Electrical cardioversion may also be used as diagnostic tool for patients with persistent AF to ascertain the value of sinus rhythm restoration on symptoms, or to assess improvement in left ventricular function.1,3
Drug therapies
What are the alternatives to long-term medication?
FIRST-LINE RHYTHM CONTROL THERAPY
Classa
Levelb
Catheter ablation is recommended as a first-line option with a shared decision-making rhythm control strategy in patients with paroxysmal AF (Atrial Fibrillation), to reduce symtoms, recurrence, and progression of AF.
1
A
AF PATIENTS RESISTANT OR INTOLERANT TO ANTIARRHYTHMIC DRUG THERAPY
Classa
Levelb
Catheter ablation is recommended in patients with paroxysmal or persistent AF resistant or intolerant to antiarrhythmic drug therapy to reduce symptoms, recurrence, and progression of AF.
1
A
PATIENTS WITH HEART FAILURE
Classa
Levelb
AF catheter ablation is recommended in patients with AF and HFrEF (Heart Failure with reduced Ejection Fraction) with high probability of tachycardia-induced cardiomyopathy to reverse left ventricular dysfunction.
1
B
Table adapted from Recommendation Table 18, 2024 ESC Guidelines for the Management of Atrial Fibrillation.1
a Class of recommendation
b Level of evidence
Ablation technology has evolved over the years to increase the precision and efficiency of the procedure and to minimise the risk of damage to healthy heart tissue or adjacent structures.
The evolution of catheter ablation technology: increasing precision, safety and effectiveness15-18
RADIOFREQUENCY ABLATION (RFA)

First used for the treatment of AF in 1991, RFA uses highfrequency energy to create extreme heat to destroy target tissue19
CRYOBALLOON ABLATION

First used for the treatment of AF in 2005, cryoballoon ablation uses extreme cold to destroy target tissue20
PULSED FIELD ABLATION (PFA)

The FARAPULSETM PFA System was approved for use in Europe in 2021.21 PFA uses high amplitude pulsed electrical fields to destroy target tissue
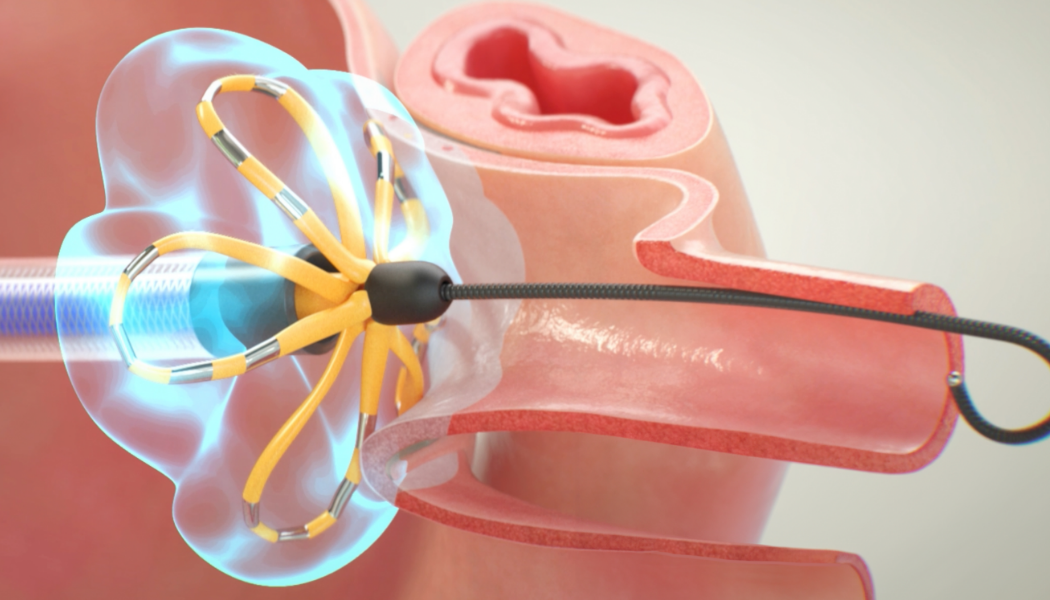
FARAPULSE PFA is backed by over 10 years of research, including 55 clinical trials and more than 150 scientific publications to date.25 One pivotal randomised controlled trial, published in the New England Journal of Medicine, found that paroxysmal AF patients treated with FARAPULSE PFA showed a 73.3% treatment success rate at one year, with antiarrhythmic medication discontinued after the three-month blanking period.15 A more recently published study has shown comparable outcomes in persistent AF patients, with a primary effectiveness event-free rate at 12 months of 73.4% with FARAPULSE.23
REFERENCES:
1. Van Gelder IC, Rienstra M, Bunting KV, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314-3414. doi:10.1093/eurheartj/ehae176.
2. Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med 2020;383(14):1305-1316. doi:10.1056/NEJMoa2019422.
3. NICE Guideline NG196. Atrial fibrillation: diagnosis and management. April 2021, updated June 2021 available at https://www.nice.org.uk/guidance/ng196/chapter/Recommendations (last accessed May 2025)
4. Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193
5. Poole JE, Bahnson TD, Monahan KH, et al. CABANA Investigators and ECG Rhythm Core Lab. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol. 2020;75(25):3105–3118. doi: 0.1016/j.jacc.2020.04.065.
6. AlTurki A, Proietti R, Dawas A, et al. Catheter ablation for atrial fibrillation in heart failure with reduce ejection fraction: a symptomatic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19(1):18. doi: 10.1186/s12872-019-0998-2.
7. Asad ZUA, Yousif Ali, Khan MS, et al. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ Arrhythm Electrophysiol. 2019;12(9):e007414. doi: 10.1161/CIRCEP.119.007414.
8. Khan SU, Rahman H, Talluri S et al. The Clinical Benefits and Mortality Reduction Associated With Catheter Ablation in Subjects With Atrial Fibrillation: A Systematic Review and Meta-Analysis JACC Clin Electrophysiol. 2018;4(5):626-635. doi: 10.1016/j.jacep.2018.03.003
9. Ruzieh M, Foy AJ, Aboujamous NM, et al. Meta-Analysis of Atrial Fibrillation Ablation in Patients with Systolic Heart Failure. Cardiovasc Ther. 2019:8181657. doi: 10.1155/2019/8181657.
10. Turagam MK, Garg J, Whang W, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170(1):41-50. doi: 10.7326/M18-0992
11. Chen C, Zhou X, Zhu M, et al. Catheter ablation versus medical therapy for patients with persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized controlled trials. J Interv Cardiovasc Electrophysiol. 2018;52(1):9-18. doi: 10.1007/s10840-018-0349-8
12. Calkins H, Hindricks G, Cappato R, et al. Heart Rhythm. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi: 10.1016/j.hrthm.2017.05.012.
13. Allan KS, Aves T, Hentry S, et al. Health-Related Quality of Life in Patients with Atrial Fibrillation Treated With Catheter Ablation or Antiarrhythmic Drug Therapy: A Systematic Review and Meta-analysis. CJC Open. 2020;2(4):286-295. doi: 10.1016/j.cjco.2020.03.013.
14. Karakasis P, Tzeis S, Pamporis K, et al. Impact of catheter ablation timing according to duration of atrial fibrillation history on arrhythmia recurrences and clinical outcomes: A meta-analysis. Europace. 2025 May 28:euaf110. doi: 10.1093/europace/euaf110. Epub ahead of print.
15. Reddy VY, Gerstenfeld EP, et al; ADVENT Investigators. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2023;389(18):1660-1671. doi: 10.1056/NEJMoa2307291.
16. Ekanem, E, Neuzil, P, Reichlin, T et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med. 2024;30(7):2020-2029 doi.org/10.1038/s41591-024-03114-3
17. Reddy VY, Mansour M, Calkins H. Pulsed Field vs Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation: Recurrent Atrial Arrhythmia Burden. J Am Coll Cardiol. 2024 Jul 2;84(1):61-74. doi: 10.1016/j.jacc.2024.05.001.
18. Della Rocca DG, Marcon L, Magnocavallo M, et al. Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: A propensity score matched comparison. Europace. 2023;26(1):euae016.
19. Ghzally Y, Ahmed I, Gerasimon G. Catheter Ablation. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470203/. Last accessed May 2025.
20. Yang H, Xiang J, Shen J, et al. Global Research Trends of Cryoablation for Atrial Fibrillation from 2002 to 2022: A Bibliometric Analysis. Anatol J Cardiol. 2023;27(12):688-696. doi: 10.14744/AnatolJCardiol.2023.
21. Cardiac Rhythm News. 29 January 2021. Available at: https://cardiacrhythmnews.com/farapulse-gains-ce-mark-for-its-pulsed-field-ablation-system/. Last accessed May 2025.
22. BSC data on file. 2025
23. Reddy VY, Gerstenfield EP, Schmidt B, et al. Pulsed Field Ablation of Persistent Atrial Fibrillation With Continuous ECG Monitoring Follow-Up: ADVANTAGE AF-Phase 2. Circulation. Published online April 2025. doi:10.1161/CIRCULATIONAHA.125.074485
24. Füting A, Neven K, Howel D et al. Patient discomfort following pulsed field ablation for paroxysmal atrial fibrillation – an assessment of chest and groin pain using the Numeric Rating Scale. Clin Res Cardiol (2021). 10.1007/s00392-021-01933-9
25. FARAPULSE Clinical Compendium.
26. ClinicalTrials.gov ID NCT06861673. COnCOmitaNt Pulse Field Ablation Based pUlmonary Vein Isolation and lefT Atrial Appendage Closure - The COCONUT Study.
27. ClinicalTrials.gov ID NCT06686485. cOncomitant Left Atrial aPpendage Closure and Pulsed Field ablaTION-Asia - OPTION-A
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.



